Introduction: Hydroxyurea has well-described clinical and laboratory benefits in sickle cell anemia (SCA). Traditionally, hemoglobin concentration (Hb) and fetal hemoglobin (HbF) are used to assess treatment response, but these aggregate parameters do not fully capture the range of erythrocyte abnormalities in SCA. Dense red blood cells (DRBCs) are defined as RBCs with density >1.11 g/mL as measured by density-gradient fractionation methods, which are tedious and require technical expertise. This subpopulation of dehydrated RBCs has been associated with increased SCA severity but has not been extensively studied in children. Here we describe the utility of rapid, automated DRBC quantitation and show that the percent of DRBCs (%DRBCs) is a robust biomarker of hydroxyurea response in children with SCA.
Methods: The Therapeutic Response Evaluation and Adherence Trial (TREAT, NCT02286154) was a single‐center prospective study that demonstrated individualized, pharmacokinetic (PK)‐guided hydroxyurea dosing in young patients (mean age 12.1 months at initiation) results in robust and sustained HbF levels >30-40% for most adherent patients. The longitudinal follow-up phase of TREAT aims to comprehensively evaluate hematologic parameters and organ function to demonstrate the long-term benefits of this treatment strategy. During clinic visits (every 3-6 months), TREAT participants had complete blood counts (CBCs) with the ADVIA® 2120i system. This automated analyzer quantifies hyperchromic RBCs within seconds by directly measuring cell hemoglobin concentration, where DRBCs are known to correspond to RBCs with a measured MCHC >41 g/dL. Data were analyzed at baseline and after at least 6 months of hydroxyurea therapy (M≥6). α- and β-globin genotypes were determined for all patients.
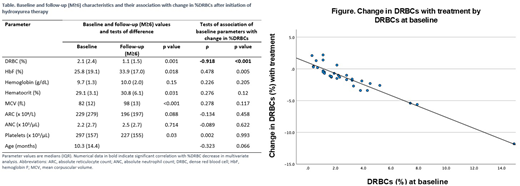
Results: Thirty-three TREAT participants had ADVIA data available for analysis (Table). All had homozygous SCA except one with sickle-β0-thalasemia; 17 (51.5%) were male. The median age at hydroxyurea initiation was 10.3 months (mean 26.1 months; range 6 months - 17 years). The median duration of hydroxyurea therapy was 27.7 months (mean 31.5). At baseline, median %DRBCs was 2.1%. Baseline %DRBCs were directly correlated with age at initiation of hydroxyurea (p=0.022) and inversely correlated with baseline HbF (p=0.001). After initiation of hydroxyurea (M≥6), there was a 47.6% reduction in %DRBCs (p=0.001). Median %DRBCs at M≥6 was directly correlated with absolute reticulocyte (ARC) (p=0.002), absolute neutrophil (ANC) (p=0.003) and platelet counts (p<0.001) and inversely correlated with Hb (p=0.013) and hematocrit (Hct) (p=0.019), consistent with the laboratory benefits of hydroxyurea. In bivariate analysis, the change in %DRBCs from baseline was directly correlated with baseline %DRBCs (p<0.001) and inversely correlated with baseline HbF (p=0.005). In multivariate analysis, baseline %DRBCs was the only biologic parameter that independently predicted %DRBC change (β=-0.86, p<0.001, model r2=0.91; Figure). Even when baseline %DRBCs were only mildly increased (as low as 1.5%), participants had a marked decline in %DRBC with hydroxyurea. When baseline %DRBCs were very low at baseline (<1.5%), they remained low after treatment. Of note, there was no correlation between %DRBC change and α-thalassemia status.
Conclusions: DRBCs can be rapidly quantified without the tedium of classical density-gradient fractionation methods using the automated ADVIA 2120i hematology analyzer, which directly measures hyperchromic RBCs. These data from TREAT demonstrate the feasibility of serial %DRBC measurements in clinical trials and clinical practice. TREAT participants had low baseline DRBCs compared to untreated adult SCA patients (reported mean ≈12%), reflecting the young age of these patients. Nevertheless, there was a significant decline in %DRBCs with treatment that correlated strongly with the known laboratory benefits of hydroxyurea. In conclusion, automated DRBC measurements are a robust biomarker that can be incorporated in a panel of laboratory measurements of response to hydroxyurea (and severity of SCA). A larger, multicenter study of PK-guided hydroxyurea therapy (HOPS, NCT03789591) is currently underway in 11 US sickle cell centers and will provide generalizable data on DRBC responses with optimal hydroxyurea therapy.
Malik:Aruvant Sciences, Forma Therapeutics, Inc.: Consultancy; Aruvant Sciences, CSL Behring: Patents & Royalties. Kalfa:Agios Pharmaceuticals, Inc: Consultancy, Research Funding; Forma Therapeutics, Inc: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal